PIPELINE
Pharmaceutical Solutions Based on
Long-acting Technology
Pipeline
이뮤노포지㈜는 반감기 연장 플랫폼 기술 기반의 파이프라인을 보유한 신약개발 회사입니다.
- Home
- >
- Pipeline
- >
- Pipeline
주요 파이프라인 현황
| Identification | Indication | Target | Discovery | Non-clinical | Phase I | Phase II | 비고 | |
|---|---|---|---|---|---|---|---|---|
| ELP Platform |
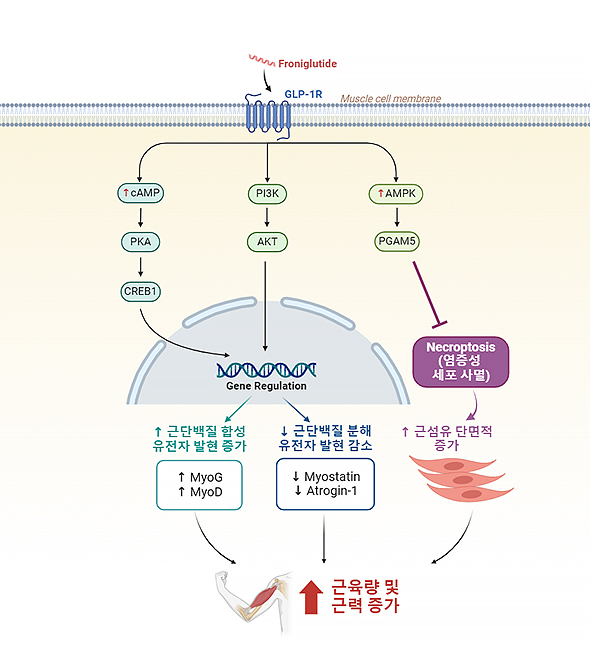
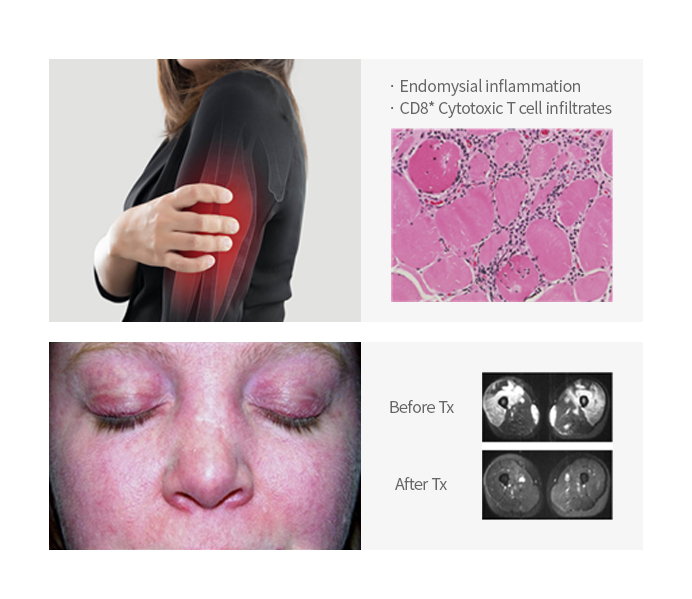
Froniglutide | Dermatomyositis (DM)/ Polymyositis (PM) |
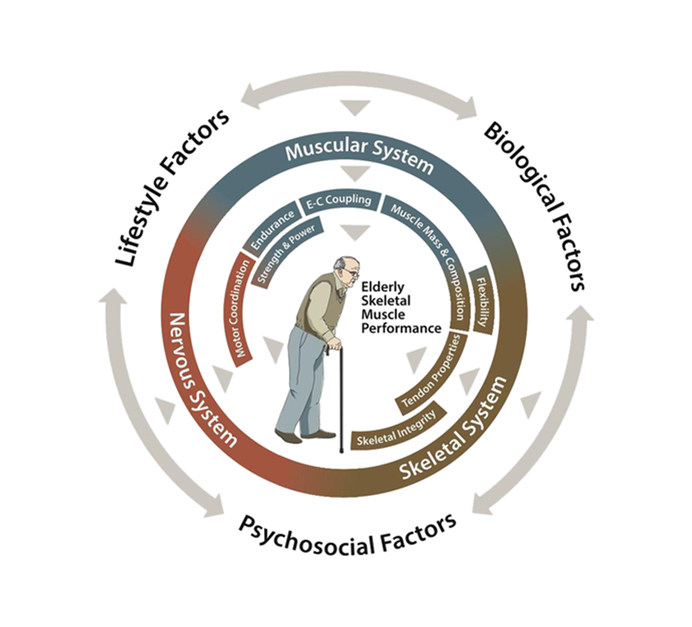
GLP-1R* (Weekly) * Sarcopenia |
|
FDA , 식약처 ODD |
|||
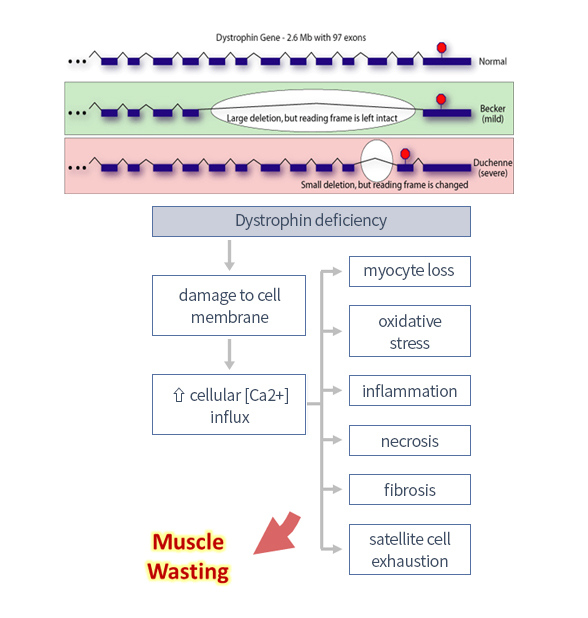
| Duchenne Muscular Dystrophy (DMD) |
|
FDA ODD | ||||||
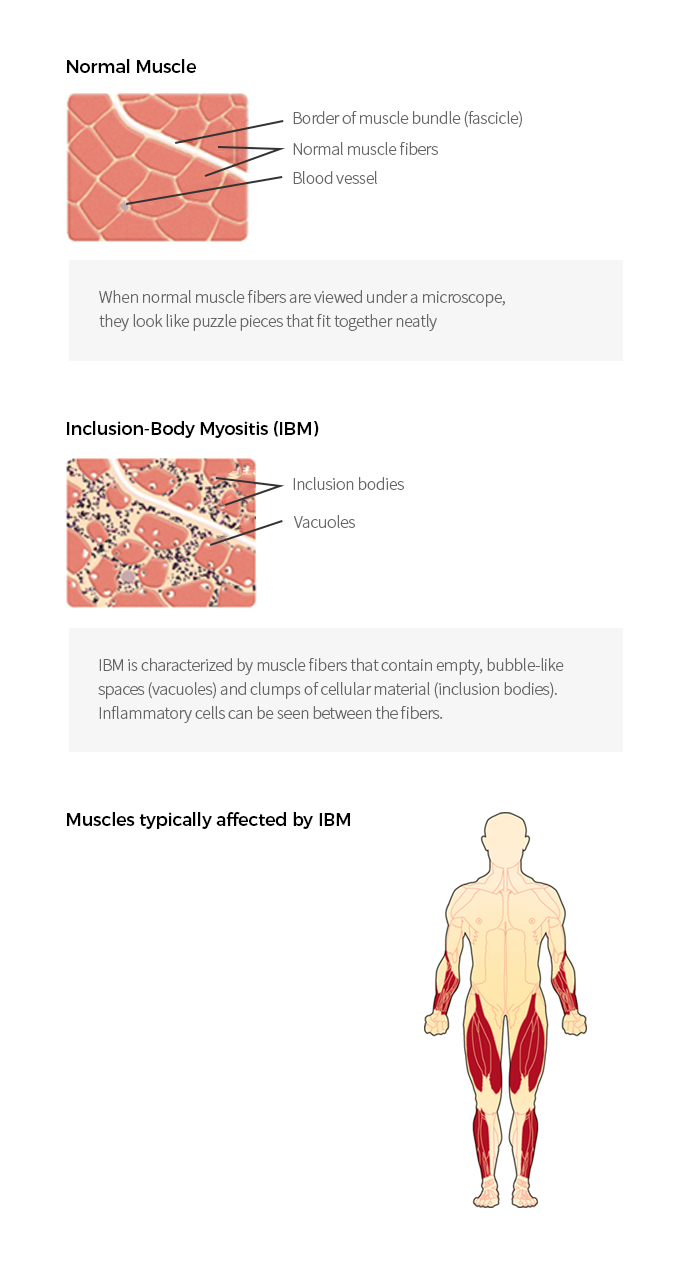
| Inclusion Body Myositis (IBM) |
|
|||||||
| Senile Sarcopenia |
|
|||||||
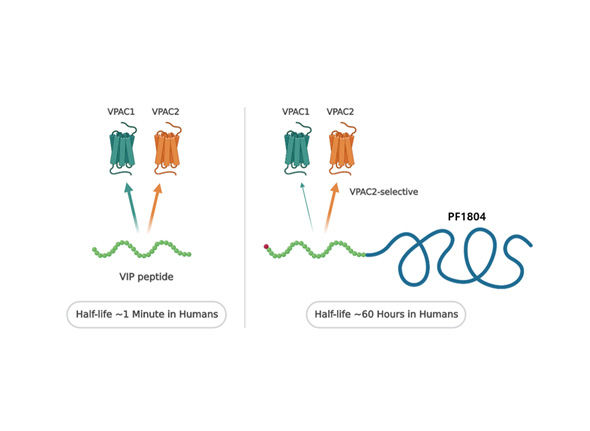
| Pemziviptadil | Cardiomyopathy(DMD), Heart Failure, Cystic Fibrosis |
VPAC2 |
|
FDA ODD | ||||
| PF1805 | Achondroplasia | NPR-B (Weekly) |
|
|||||
| PF1806 | Short Bowel Syndrome (SBS) | GLP-2R (Weekly) |
|
|||||
| PF1807 | Sarcopenia | GLP-1R (Monthly) |
|
|||||
| PF1808 | L/O |
|
||||||
| PF1802 | Amyotrophic Lateral Sclerosis (ALS), Parkinson’s Disease |
Bifunctional |
|
|||||
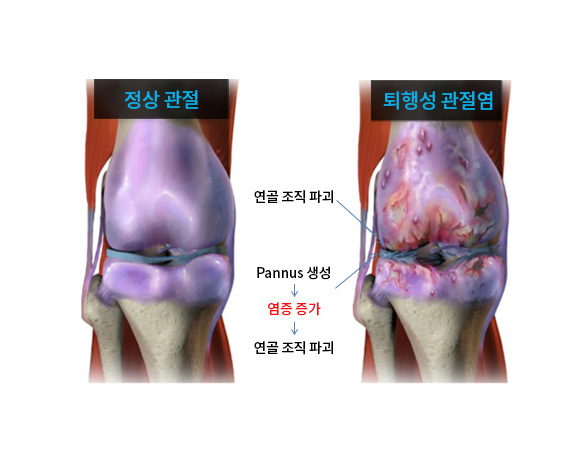
| PF1803 | Osteoarthritis (OA) | Bifunctional OSCAR + |
|
|||||
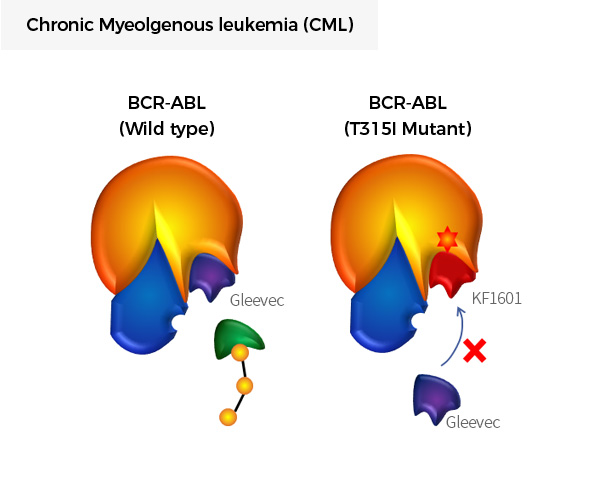
| KF1601 | Drug Resistant Chronic Myeloid Leukemia (CML) |
BCR-ABL1 (T315I) |
|
FDA ODD | ||||